An international Europe Uterine Cancer Diagnostics Market report has an assessment of the factors influencing the supply and demand of the related products and services, and challenges reckoned by market players. The report starts with a market outlook together with the data integration and capabilities study with the appropriate findings. It has projected strong upcoming growth of the market. This market research report combining secondary research which includes reference to different statistical databases, related patent and regulatory bibliography and a number of internal and external proprietary information. With the help of key information and market insights from technical and marketing experts, the report provides an objective estimation of the EUROPE UTERINE CANCER DIAGNOSTICS market.
The significant EUROPE UTERINE CANCER DIAGNOSTICS market report sheds light on each region, market size in terms of (USD Mn) for every individual segment and their sub-segment for the period from 2023 to 2030, considering the macro and micro situation factors. The document gives a brief introduction to the research report outlook, TOC, list of tables and figures, an outlook to key players of the market and comprising key regions. An extensive summary of the EUROPE UTERINE CANCER DIAGNOSTICS market comprises prominent market that includes several major market leaders in the report. The hand-picked EUROPE UTERINE CANCER DIAGNOSTICS market survey report necessitates an in-depth analysis pertaining to the product manufactured by the vendors combining with the product application range.
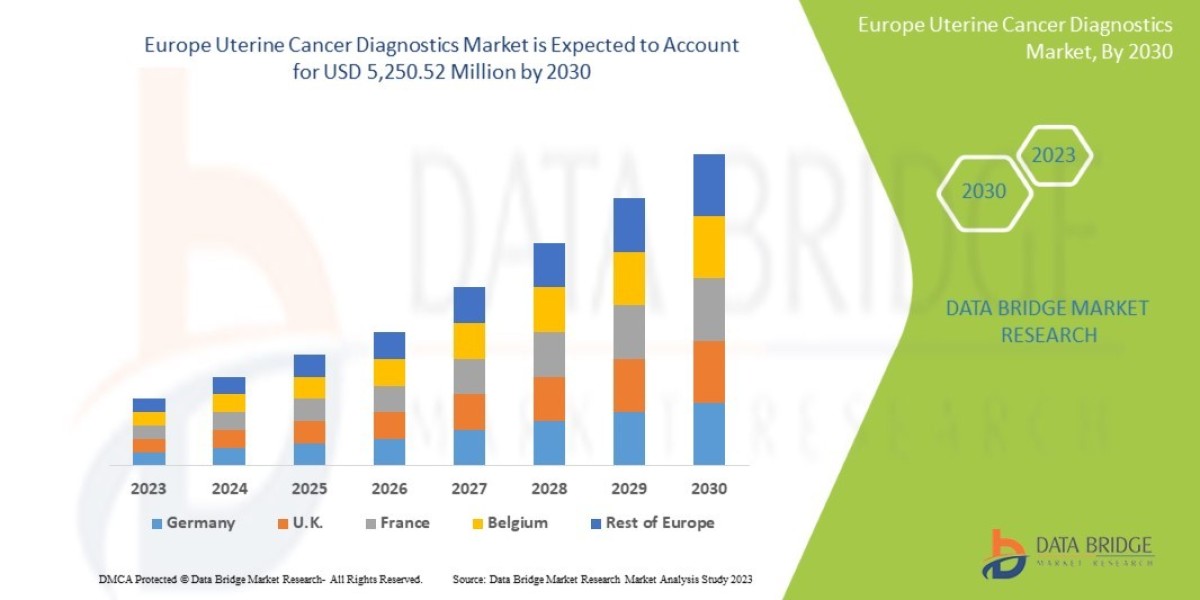
Data Bridge Market Research analyzes that the Europe uterine cancer diagnostics market is expected to reach the value of USD 5,250.52 million by 2030, at a CAGR of 10.5% during the forecast period.
Download Sample PDF Copy of this Report to understand structure of the complete report (Including Full TOC, Table Figures) @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=europe-uterine-cancer-diagnostics-market
Market Overview
Diagnosis of uterine cancer includes ultrasound, biopsy procedures and blood tests. Ultrasound tests use sound waves and produce images of the ovaries and uterus. Two types of ultrasound are included in diagnostic testing for uterine cancer: abdominal ultrasound and transvaginal ultrasound. An abdominal scan test involves scanning the full bladder with a small probe across the abdomen, while a transvaginal scan involves inserting a probe tube into the vagina to scan or create an image. Biopsy procedures include endometrial biopsy, hysteroscopy and dilation and curettage.
Cancer is one of the leading causes of death worldwide and the prevalence of this disease has increased at an alarming rate. Therefore, health professionals are focusing on developing effective screening and treatment solutions to control obesity rates. Moreover, an endometrial biopsy involves removing some malignant tissue from the endometrium and sending it for a testing procedure. The growing number of geriatric women in the U.S. is also a major factor contributing to the growth of the uterine cancer diagnostic market.
Drivers
Technological advancements in uterine cancer diagnostics
Uterine cancer is a ubiquitous gynaecological disease with many tests available for diagnosis, which include pelvic examination, endometrial biopsy, Dilation and Curettage (DC), Transvaginal ultrasound, Computed Tomography (CT or CAT) scan, Magnetic Resonance Imaging (MRI) and biomarker testing of the tumor. Traditional means of detecting and monitoring cancers rely largely on imaging and, where possible, blood-based protein biomarkers, many of which are non-specific. Most cases of uterine cancer are diagnosed at late stages, as the presenting symptoms are often non-specific. The signs and symptoms of uterine cancer vary from person to person, making it difficult to provide accurate early diagnosis. This calls for personalized diagnostic plans, which involve multiple diagnostic tests.
Thus, technological advancements in uterine cancer diagnostics are expected to drive the market's growth.
Global Europe Uterine Cancer Diagnostics Market Scope and Market Size
Europe uterine cancer diagnostics market is segmented into diagnostic type, type, age group, end user and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
BY DIAGNOSTIC TYPE
Instrument Based
Procedure Based
On the basis of diagnostic type, the Europe uterine cancer diagnostic market is segmented into instrument based and procedure based.
BY TYPE
Endometrial Cancer
Uterine Sarcoma
On the basis of type, the Europe uterine cancer diagnostic market is segmented into endometrial cancer and uterine sarcoma.
BY AGE GROUP
30 years
31-40 years
41-50 years
51-60 years
60 years
On the basis of age group, the Europe uterine cancer diagnostic market is segmented into 30, 31-40, 41-50, 51-60 and 60.
BY END USER
Hospitals
Diagnostic Centers
Cancer Research Centers
Ambulatory Surgical Centers
Specialized Clinics
Others
On the basis of end user, the Europe uterine cancer diagnostic market is segmented into hospitals, diagnostic centers, cancer research centers, ambulatory surgical centers, specialized clinics and others.
BY DISTRIBUTION CHANNEL
Direct Tenders
Third Party Distributors
Others
On the basis of distribution channel, the Europe uterine cancer diagnostic market is segmented into direct tender, third party distributors and others.
Some of the major players operating in the Europe uterine cancer diagnostics market are F-Hoffmann La Roche Ltd., Siemens Healthcare Private Limited, Narang Medical Limited, ESAOTE SPA, Olympus Corporation, Integra LifeSciences, Canon Medical Systems ANZ Pty Limited., KARL STORZ SE Co. KG, Stryker, Guzip Biomarkers Corporation, General Electric Company, FUJIFILM Corporation, Koninklijke Philips N.V., GRAIL, B. Braun SE, and Arquer Diagnostics Ltd. among others.
Get the Full Table of Contents @
https://www.databridgemarketresearch.com/toc/?dbmr=europe-uterine-cancer-diagnostics-market
Browse Trending Reports:
About Data Bridge Market Research:
An absolute way to predict what the future holds is to understand the current trend! Data Bridge Market Research presented itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are committed to uncovering the best market opportunities and nurturing effective information for your business to thrive in the marketplace. Data Bridge strives to provide appropriate solutions to complex business challenges and initiates an effortless decision-making process. Data Bridge is a set of pure wisdom and experience that was formulated and framed in 2015 in Pune.
Data Bridge Market Research has more than 500 analysts working in different industries. We have served more than 40% of the Fortune 500 companies globally and have a network of more than 5,000 clients worldwide. Data Bridge is an expert in creating satisfied customers who trust our services and trust our hard work with certainty. We are pleased with our glorious 99.9% customer satisfaction rating.
Contact Us: -
Data Bridge Market Research
US: +1 888 387 2818
United Kingdom: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]