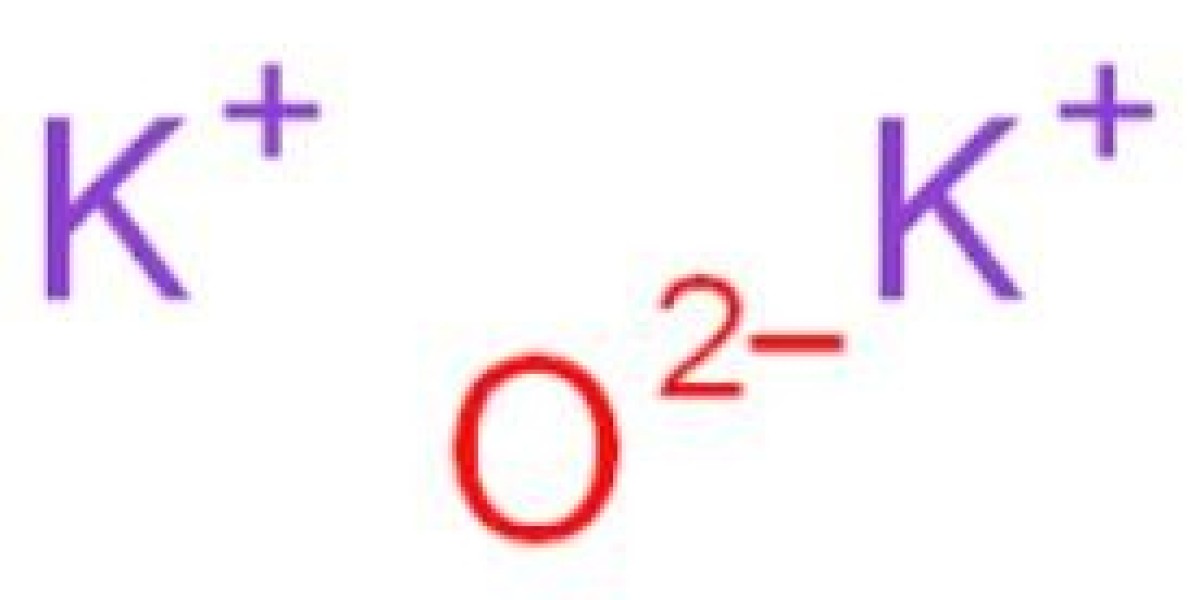
Potassium oxide consists of potassium and oxygen, which are connected together by ionic bonds. The Oxidation state of potassium is+1. So it's easy to lose an electron. Potassium is an alkali metal. It has a high tendency to combine with any other counter ions to complete its valence state. Therefore, it has high reactivity in free form. It easily reacts with oxygen to form potassium hydroxide. It is light yellow and widely used as fertilizer. It is a highly corrosive alkali when dissolved in water. Here, we will study the formula, structure, physical and chemical properties and uses of Potassium oxide. When potassium oxide is dissolved in water, it is a strong corrosive alkali.
structure
The Potassium oxide formula contains two potassium atoms and one oxygen atom. These atoms are connected together by bonds. Potassium is in+1 Oxidation state. The Oxidation state of oxygen is -2. To balance the valence, two potassium atoms combine with one oxygen atom. Therefore, the chemical formula of Potassium oxide is K2O
physical characteristics
It is solid and has a light yellow color.
The molecular weight is 94.2 grams/mole.
The density of K2O is 2.35 grams per cubic centimeter.
The melting point of Potassium oxide is 740 ℃.
It is soluble in ether and ethanol.
chemical property
Potassium burns (O2) in the atmosphere to produce Potassium oxide.
4K+oxygen 2 square kilometers
When potassium hydroxide is treated with water, it forms potassium hydroxide.
K2O+H2O Science
When it reacts with strong acids, it forms salts and water.
K2O+hydrochloric acid Potassium chloride+water
Potassium can be directly added to water
2 kilometers+2 hours 2 hours+2 hours
Application and purpose
It is used as fertilizer in agriculture.
It is insoluble in water and highly stable. This makes it very useful in the ceramic industry. It is used to manufacture lightweight bowls and structural compounds in aerospace.
It is used to prepare soap and glass. It is commonly referred to as pure potassium fertilizer.
It is used to treat fungal infections, such as zygomatic bacteria
It is also used to treat animal related diseases.
conclusion
This paper introduces the chemical formula, physical properties and application of Potassium oxide and Potassium oxide. Potassium oxide is a Ionic compound. After acid treatment, it forms salt and water. It is also used as fertilizer in agriculture. May be toxic when inhaled and ingested. It is suitable for the ceramic, glass, and optical industries.
Do you know?
It must be noted that the chemical formula of Potassium oxide is K2O. Superoxide potassium is a Inorganic compound with chemical formula of KO2. It is a yellow Paramagnetism solid that decomposes in humid air. The Oxidation state of oxygen in Superoxide potassium is calculated as -1. These two compounds are completely different in chemical and physical properties.