Key Market Insights
The current market landscape features the presence of over 90 players engaged in the smart pharmaceutical and healthcare labels domain, worldwide
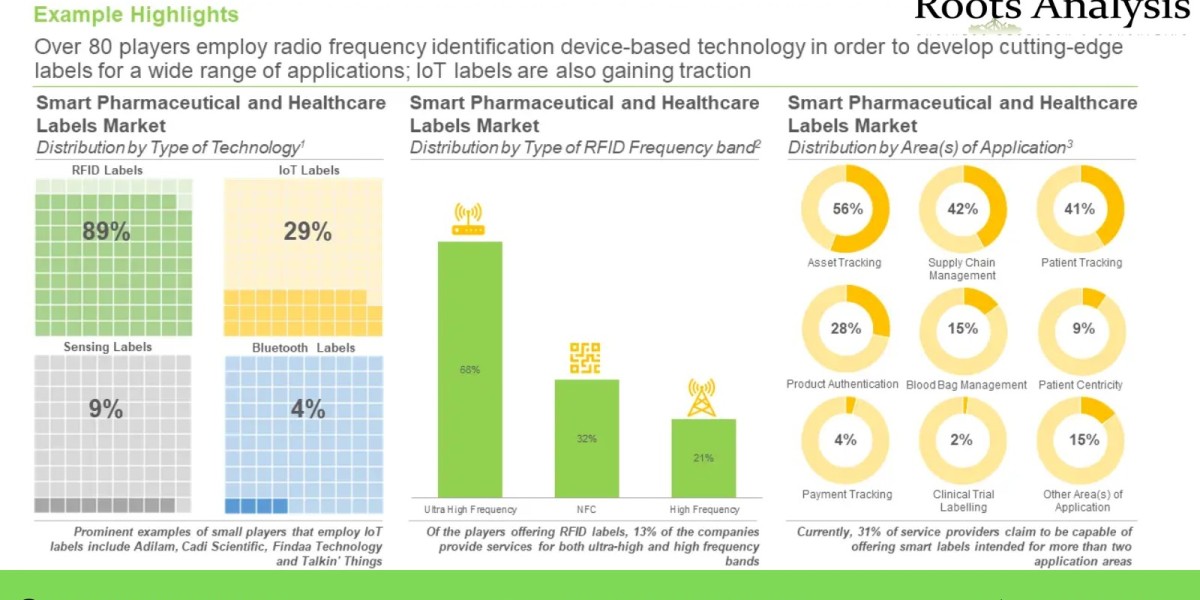
Over 80 players employ radio frequency identification device-based technology in order to develop cutting-edge labels for a wide range of applications; IoT labels are also gaining traction
Smart labels providers are continuously upgrading their existing portfolio and are seeking investments, as well as expanding their intellectual capital, in order to gain an edge in this competitive industry
More than 400 patents have been granted / filed for smart pharmaceutical and healthcare labels, primarily led by industry players based in North America
A rise in interest in partnerships, in recent past, involving both international and indigenous stakeholders, validate the growing interest in this domain; maximum number of such deals were signed in 2019
Based on the pioneer-migrator-settler map, we have classified the smart label providers into different categories; a selection of pioneers is expected to provide valuable offerings to lead the market in the longer term
The Smart labels market is expected to witness a healthy growth of over 15% in the coming decade; the opportunity is likely to be well distributed across various technologies and different geographical regions
In long term, the majority share of revenues is likely to be driven by players offering smart labels for primary packaging to prevent counterfeiting of drugs
Request a free sample of the report
https://www.rootsanalysis.com/reports/smart-labels-market/request-sample.html
Table of Contents
1. PREFACE
1.1. Scope of the Report
1.2. Market Segmentations
1.3. Research Methodology
1.4. Frequently Asked Questions
1.5 Chapter Outlines
2. EXECUTIVE SUMMARY
2.1. Chapter Overview
3. INTRODUCTION
3.1. Chapter Overview
3.2. Role of Smart Labels in the Healthcare Sector
3.2.1 Types of Smart Labels
3.2.1.1. Radio Frequency Identification Device (RFID)
3.2.1.2. Near-Field Communication (NFC)
3.2.1.3. Internet of Things (IoT)
3.2.2. Need for Smart Labels in the Healthcare Sector
3.2.3. Advantages of Using Smart Labels in Healthcare Sector
3.3. Roadblocks and Challenges Associated with the Adoption of Smart Labels
3.4. Adoption of Smart Labels in the Healthcare Sector
3.4.1. Key Drivers for Adoption of Smart Labels
3.5. Future Outlook
4. MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Smart Pharmaceutical and Healthcare Label Providers: Overall Market Landscape
4.2.1. Analysis by Year of Establishment
4.2.2. Analysis by Company Size
4.2.3. Analysis by Location of Headquarters
4.2.4. Analysis by Company Size and Location of Headquarters
4.2.5. Analysis by Type of Technology
4.2.6. Analysis by Company Size and Type of Technology
4.2.7. Analysis by Type of RFID Frequency Band
4.2.8. Analysis by Area(s) of Application
4.2.9. Analysis by Company Size and Area(s) of Application
4.2.10. Analysis by Type of Technology and Area(s) of Application
5. COMPANY COMPETITIVENESS ANALYSIS
5.1. Chapter Overview
5.2. Assumptions / Key parameter
5.3. Methodology
5.3.1. Smart Pharmaceutical and Healthcare Label Providers based in North America
5.3.2. Smart Pharmaceutical and Healthcare Label Providers based in Europe
5.3.3. Smart Pharmaceutical and Healthcare Label Providers based in Asia-Pacific and RoW
5.4. Company Competitiveness Analysis: Benchmarking the Capabilities of Leading Players
6. COMPANY PROFILES
6.1. Chapter Overview
6.2. CCL Industries
6.2.1. Company Overview
6.2.2. Financial Information
6.2.3. Area(s) of Application
6.2.4. Recent Developments and Future Outlook
6.3. Schreiner
6.3.1. Company Overview
6.3.2. Area(s) of Application
6.3.3. Recent Developments and Future Outlook
6.4. Datalogic
6.4.1. Company Overview
6.4.2. Financial Information
6.4.3. Area(s) of Application
6.4.4. Recent Developments and Future Outlook
6.5. Tadbik
6.5.1. Company Overview
6.5.2. Area(s) of Application
6.5.3. Recent Developments and Future Outlook
6.6. SATO Asia Pacific
6.6.1. Company Overview
6.6.2. Financial Information
6.6.3. Area(s) of Application
6.6.4. Recent Developments and Future Outlook
6.7. Invengo
6.7.1. Company Overview
6.7.2. Area(s) of Application
6.7.3. Recent Developments and Future Outlook
6.8. Intellhydro Technology
6.8.1. Company Overview
6.8.2. Area(s) of Application
6.8.3. Recent Developments and Future Outlook
6.9. RFiD Discovery
6.9.1. Company Overview
6.9.2. Area(s) of Application
6.9.3. Recent Developments and Future Outlook
6.10. ID Tech Solutions
6.10.1. Company Overview
6.10.2. Area(s) of Application
6.10.3. Recent Developments and Future Outlook
7. PATENT ANALYSIS
7.1. Chapter Overview
7.2. Scope and Methodology
7.3. Smart Pharmaceutical and Healthcare Labels: Patent Analysis
7.3.1. Analysis by Publication Year
7.3.2. Analysis by Publication Year and Type of Patent
7.3.3. Analysis by CPC Code
7.3.4. Analysis by Type of Applicant
7.3.5. Analysis by Geography
7.3.6. Analysis by Emerging Focus Areas
7.3.7. Leading Industry Players: Analysis by Number of Patents
7.4. Smart Pharmaceutical and Healthcare Labels: Patent Benchmarking Analysis
7.4.1. Analysis by Patent Characteristics
7.5. Smart Pharmaceutical and Healthcare Labels: Patent Valuation Analysis
8. PARTNERSHIPS AND COLLABORATIONS
8.1. Chapter Overview
8.2. Partnership Models
8.3. Smart Pharmaceutical and Healthcare Labels: Recent Partnerships and Collaborations
8.3.1. Analysis by Year of Partnership
8.3.2. Analysis by Type of Partnership
8.3.3.