Key Inclusions
- A general overview of novel antibody therapies including information on the different types of novel antibody therapies. In addition, the chapter presents details on the novel antibody therapies (antibody-directed enzyme prodrug therapy, TCR like antibodies, radioisotope immunoconjugates, immunotoxins, intracellular antibody and immunocytokines). It also features information on developmental approaches of novel antibody therapies, limitations, and future of novel antibody therapies
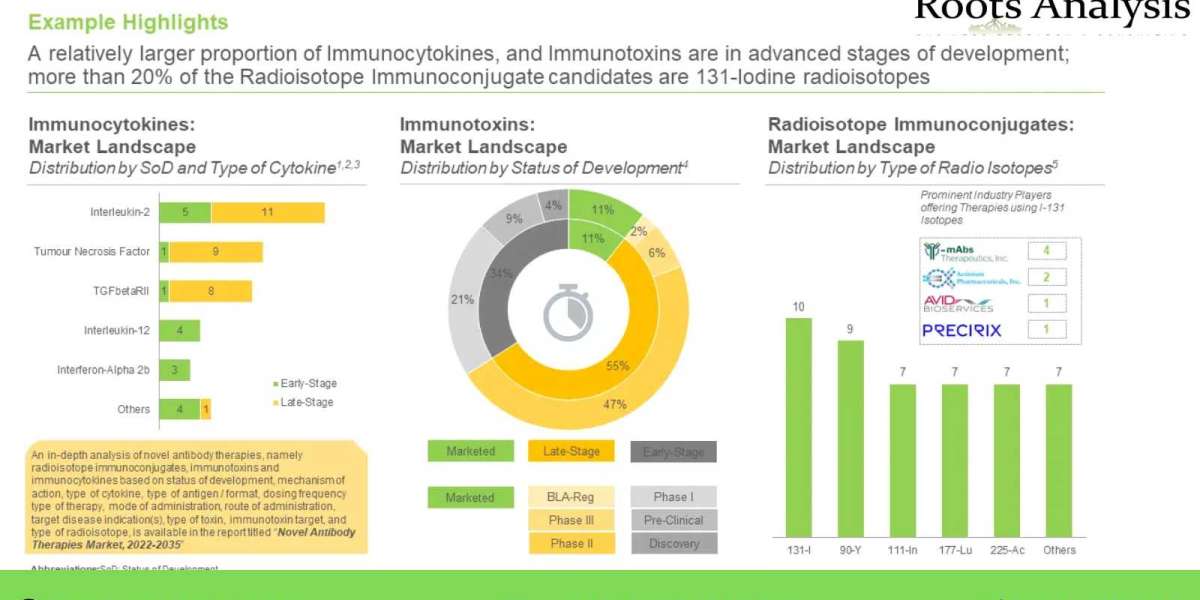
- A detailed review of the current market landscape of different types of novel antibody therapies, namely immunocytokines, based on several relevant parameters such as status of development (phase III, phase II, phase I and preclinical stage), mechanism of action (inhibition, modulation and stimulation), type of cytokine, type of antigen / format, dosing frequency, type of therapy (monotherapy and combination therapy), mode of administration (injection and infusion), route of administration (intravenous, intratumoral and subcutaneous), type of therapy and target disease indication(s).
- A detailed review of the current market landscape of different types of novel antibody therapies, namely immunotoxin based on several relevant parameters such as status of development (marketed, BLA registration, phase III, phase II phase I and preclinical stage), mechanism of action (inhibition, modulation and stimulation), type of toxin, dosing frequency, type of therapy (monotherapy and combination therapy), mode of administration (injection and infusion), immunotoxin target, route of administration (intravenous, intravesical, intraperitoneal, intratumoral and intrathecal), and target disease indication(s).
- A detailed review of the current market landscape of different types of novel antibody therapies, namely radioisotope immunoconjugates based on several relevant parameters such as status of development (marketed, phase III, phase II phase I and preclinical stage), type of radioisotope, route of administration (Intravenous, intracerebroventricular and intraperitoneal), type of therapy (monotherapy and combination therapy), dosing frequency, mode of administration (injection and infusion) and target disease indication(s). In addition, the chapter includes a list of novel antibody therapies with special designations. Further, the chapter presents a list of players developing novel antibody therapies along with information on their year of establishment, company size and location of headquarters.
- Elaborate profiles of the key players engaged in the development of novel antibody therapies (shortlisted on the basis of number of drugs). Each profile features a brief overview of the company, its financial information (if available), details on its product portfolio and a section on recent developments and an informed future outlook.
- An in-depth analysis of completed, ongoing and planned clinical studies of various novel antibody therapies, highlighting prevalent trends across different parameters, such as current trial status, trial registration year, trial phase, enrolled patient population, type of sponsor / collaborator, study design, leading industry and non-industry players (in terms of number of trials conducted), key indication(s), type of treatment, type of drug, emerging focus areas and regional distribution of trials.
- A detailed review of more than 570 peer-reviewed, scientific articles related to research on novel antibody therapies, which have been published since 2017, based on parameters, such as year of publication, type of article, emerging focus areas, top authors, key journals, most popular publisher and copyright holders.
- An insightful analysis of the patents filed / granted for novel antibody therapies, between 2017- 2021, based on various relevant parameters, such as patent publication year, type of patent, geographical location, CPC symbols, type of applicant, patent age, emerging focus areas, leading individual assignees, industry and non-industry players (in terms of number of patents granted / filed) and patent characteristics. In addition, the chapter includes a detailed patent benchmarking and an insightful valuation analysis.
- An analysis of the partnerships that have been established in this domain since 2018, covering instances of acquisition, clinical trial agreement, commercialization agreement, distribution agreement, manufacturing agreement, product development agreement, product development and commercialization agreement, research and development agreement, supply agreement and other relevant type of deals.
- An analysis of big pharma players engaged in the field of novel antibody therapies, featuring various insightful representations, such as spider web analysis, Harvey ball analysis and wind rose chart, based on several relevant parameters, such as number of therapeutics under development, type of novel antibody, status of development, number of target indications, number of partnerships, number of patents and years of experience.
The report also features the likely distribution of the current and forecasted opportunity across important market segments, mentioned below:
- Target Disease Indication(s)
- Acute Myeloid Leukemia
- Advanced / Metastatic soft-tissue sarcoma
- Melanoma
- Diffuse Large B-cell Lymphoma
- Bladder Cancer
- Prostate Cancer
- Graft-versus-host disease
- Type of Novel Antibody
- Immunocytokines
- Immunotoxins
- Radioisotope immunoconjugates
- Type of Therapy
- Combination Therapy
- Monotherapy
- Route of Administration
- Intravenous
- Intravesical
- Intratumoral
- Geographical Region
- North America
- Europe
- Asia-Pacific
Key Questions Answered
- Who are the leading industry and non-industry players engaged in the development of novel antibody therapies?
- Which novel antibody therapies are being developed across early and late stages of development?
- Which geographies are the most active in conducting clinical trials related to novel antibody therapies?
- What is the focus area of various publications related to the novel antibody therapies?
- Which are the leading funding institutes / centers supporting the research related to novel antibody therapies?
- What kind of partnership models are commonly adopted by industry stakeholders?
- What are the different initiatives undertaken by big pharma players for the development of novel antibody therapies in the recent past?
- How is the current and future market opportunity, related to novel antibody therapies likely to be distributed across key market segments?
To view more details on this report, click on the link:
https://www.rootsanalysis.com/reports/novel-antibody-therapies-market.html
You may also be interested in the following titles:
Blockchain Technology in Healthcare Market
You may also like to learn what our experts are sharing in Roots educational series:
CAR-T Cell Therapies: Addressing Key Unmet Needs Across Various Oncological Indications
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
[email protected]